In recent years, researchers have found that there has been a serious "repeated crisis" in antibody related research.
According to the statistics of Nature, 80% of researchers can't repeat other researcher's experimental results, and 60% can't repeat their own experimental results.
The quality problem of antibody is one of the main culprits leading to the unrepeatable experiment. The human protein atlas project has tested 9,000 antibody products from 29 suppliers, and more than 50% of them fail to work effectively.
It is estimated that US $350 million and US $800 million are wasted annually on the use of unidentified antibodies in the United States and around the world, such antibodies can lead to experimental failure or unreliable results.
The quality of antibody is one of the main reasons leading to unrepeatable experiments.
At present, the American Society of Pathologists and Laboratory Quality Center released a guideline on the analysis and verification principles of IHC testing (Principles of Analytic Validation of Immunohistochemical Assays), at the CAP2014 annual meeting in the United States. It was recently published in the Arch Pathol Lab Med., the article clearly pointed out the necessity of routine IHC testing for cytology samples.
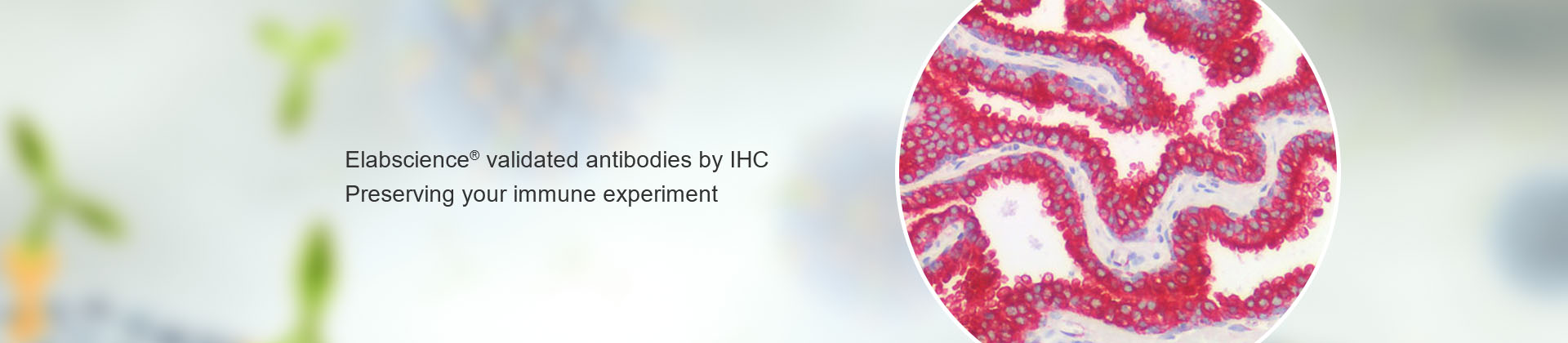
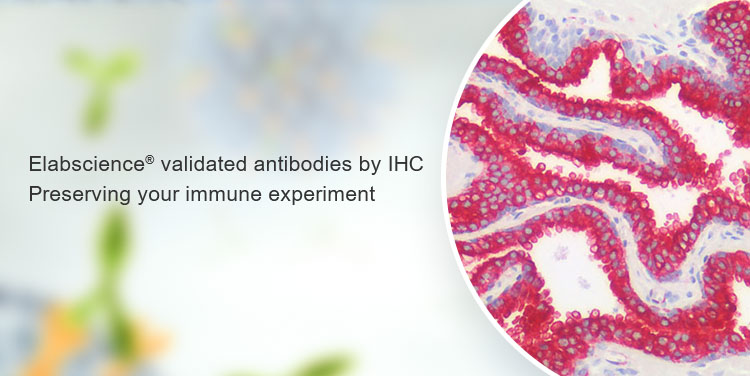
In scientific research, different scientific researchers have slight differences in operating methods, reagent configuration, and experimental details. In addition, the poor repeatability of antibodies can easily cause different degrees of incompatibility and cause uncontrollable experimental results. In order to minimize this uncontrollability, Elabscience® combined with Lifespan's immunohistochemical quality inspection platform to perform a secondary immunohistochemical quality inspection on the antibodies produced by Elabscience®. To screen high-quality immunohistochemistry antibodies according to Lifespan's quality inspection standards, and provide more professional inspections for your immunohistochemistry experiments. In addition, Elabscience® provides you with a complete set of immunohistochemistry solutions, one-stop related reagents to solve your problems.