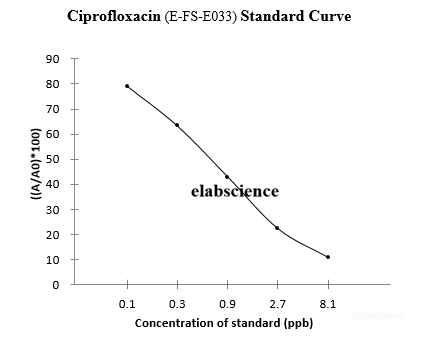
Ciprofloxacin belongs to the and has the same antibacterial spectrum as norfloxacin. Among the widely used fluoroquinolone drugs, its antibacterial activity is the strongest. In addition to high antibacterial activity against gram-negative bacilli, it also has good antibacterial effect against Staphylococcus aureus. The residues of ciprofloxacin in animal derived food lead to accumulation of drugs in the human bodyand affect the treatment of the human body.
Compared with traditional methods,the Elabscience Ciprofloxacin kit is simple, fast, and sensitive, and is suitable for the detection of large quantities of samples.
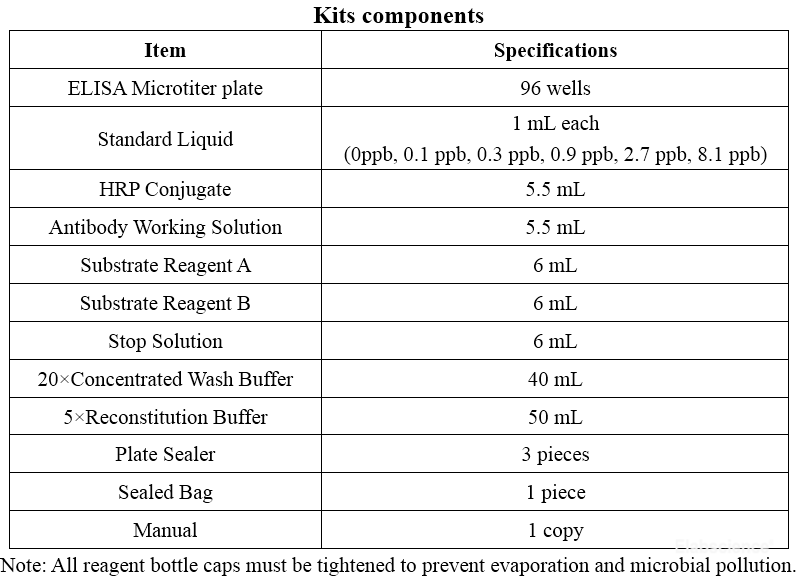
Elabscience food safety kits are used to detect hormone residues, mycotoxins, toxic and harmful substances and antibiotic residues in feed, food and other sample. It can be widely used in food and drug Administration, health departments, various colleges, scientific research units, agricultural departments, animal husbandry and veterinarians, breeding farms, slaughterhouses, food and meat products deep processing enterprises, inspection and quarantine departments, etc. It can meet the high requirements of consumers for food quality and the scientific researchers to obtain efficient experimental data. The kit is manufactured in accordance with the international quality standard ISO9001.